CAVIFAST2® Cavitation

The CAVIFAST 2 (CE Medical Class IIb) is a low frequency ultrasound device with a cavitational effect. This DERMEO device reduces the volume of the treated area by lipolysis (fat cell volume reduction) and lipoclasia (rupture of the membrane of the adipocytes) in fat cells, which leads to a loss in centimeters and an improvement of the quality of the skin.
The CAVIFAST2 device is based on low frequency ultrasounds (about 38kHz +/- 2kHz).
The CAVIFAST2 device, designed for professionals in the medical and aesthetic industry, is a latest generation cavitation device with an efficiency and safety that have been proven by dermatologists, and by a clinical study on the remains of abdominoplasty which have been kept alive, at the hospital la PITIE SALPETRIERE in Paris.
Quick, effective, and non-invasive treatments. The results are visible and measurable from the first session.
Transducers
This new generation of devices provides: see more...Generator and software
This new generation of ultrasound devices includes: see more...French manufacturing and international certifications
see more...Technology
Low frequency ultrasound technology
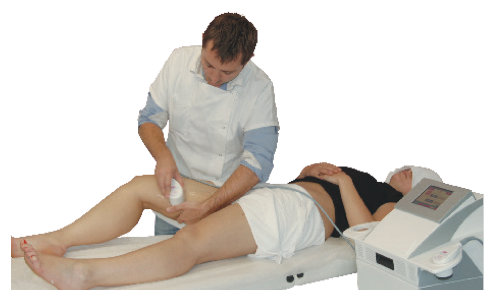
The CAVIFAST 2 device emits low frequency ultrasounds onto the skin's surface, which penetrate up to 4 cm. This depth corresponds to an action in the innermost layer of the skin: the hypodermis layer, containing the fat reserves in the body. Ultrasounds are emitted by means of handheld transducers, also known as hand pieces. They convert electrical energy into ultrasonic mechanical energy.
A transducer is composed of two pieces of ceramic, adjusted so they do not overheat. Upon activation of the device, the two pieces of ceramic vibrate simultaneously but each at a different frequency, thus creating ultrasounds.
Treatments
The CAVIFAST 2 device (CE Medical Class IIb) emits low frequency ultrasounds with a cavitational effect. This DERMEO device reduces the volume of the treated area by lipolysis (fat cell volume reduction) and lipoclasia (rupture of the membrane of the adipocytes) in fat cells, which lead to a loss in centimeters and an improvement of the quality of the skin.
Cavitation is the emission of ultrasounds within a liquid environment. When the liquid is submitted to a high intensity pressure ultrasound wave, micro-cavity bubbles are formed and their volume increases until they implode.
The generated thermal energy during this phenomenon and the compressing and decompressing waves, are responsible for the implosion of the micro-cavities.
The cavitation principle allows the treatment of excess localized fat and reduces the built-up layers in order to lose centimeters from the treated zone. Low frequency ultrasounds generated by the transducers use the water located in our cells to form bubbles.
These bubbles increase in volume until they implode, thus weakening the membrane of the adipocytes and releasing the triglycerides.
Cavitation provides a loss in centimetres of the treated area with visible results from the first session. It is important to stay hydrated before, during and after the session to facilitate the release of fat.
Protocol
Cavitation can treat fatty areas such as :
- Arms
- The abdomen
- The hips (love handles)
- Top of the thighs (saddle bags)
- The buttocks
- Thighs
Length of treatment: 10 minutes per zone of 10 cm x10 cm.
For optimal treatment, 5 to 6 sessions may be necessary.
Proven results
For all donors, the results of the study showed an increased level of glycerol which was nearly twice the level of the glycerol release of the untreated control skin, after only 10 minutes of treatment with the CAVIFAST 2.
According to our clinical study conducted by a medical specialist and a clinical researcher on volunteer patients, all subjects who volunteered for slimming treatments showed excellent results that were visible from the first session.
Clinical Study
Scientific studies of the cavitation effect
A clinical study was conducted by the GREDECO laboratory to demonstrate the quality and efficiency of the DERMEO® device. This study was carried out on fragments of human skin, by researchers of the GREDECO laboratory in the Pitié-Salpêtrière hospital in Paris.
Histological cuts of these skin fragments showed a non-invasive lipoclasia and no alteration of the dermo-hypodermic junction.
Compliance with the essential structures of the skin such as the epidermis, dermis and blood vessels demonstrates the perfect safety of treatments with the CAVIFAST2.
Glycerol release
For all donors, the results of the study showed an increased level of glycerol which was nearly twice the level of the glycerol release of the untreated control skin, after only 10 minutes of treatment with the CAVIFAST 2.
A clinical study was conducted by a clinical research associate, and a Doctor of Medicine with the DERMEO CAVIFAST 2 device. Performed on volunteer patients, visible and very satisfactory results were observed from the first session. The patients treated were aged 20-67 years and they all had a very good tolerance to ultrasonic cavitation technology and demontrated a measurable circumference reduction from the first treatment.
Videos
* The products and treatments proposed may be subject to specific regulations. Users or purchasers are responsible for verifying the regulations applicable in the country of use, as well as the specific conditions of use of this equipment with the correct authorities and their distributor.
In the United States of America the authorized claims by the F.D.A. for our device are: removal of unwanted hair from phototype skin I to V, treatment of benign cutaneous vascular lesions from phototype skin I to III, treatment of benign pigmented epidermal and cutaneous lesions from phototype skin I to III, treatment of inflammatory acne from phototype skin I to III.















